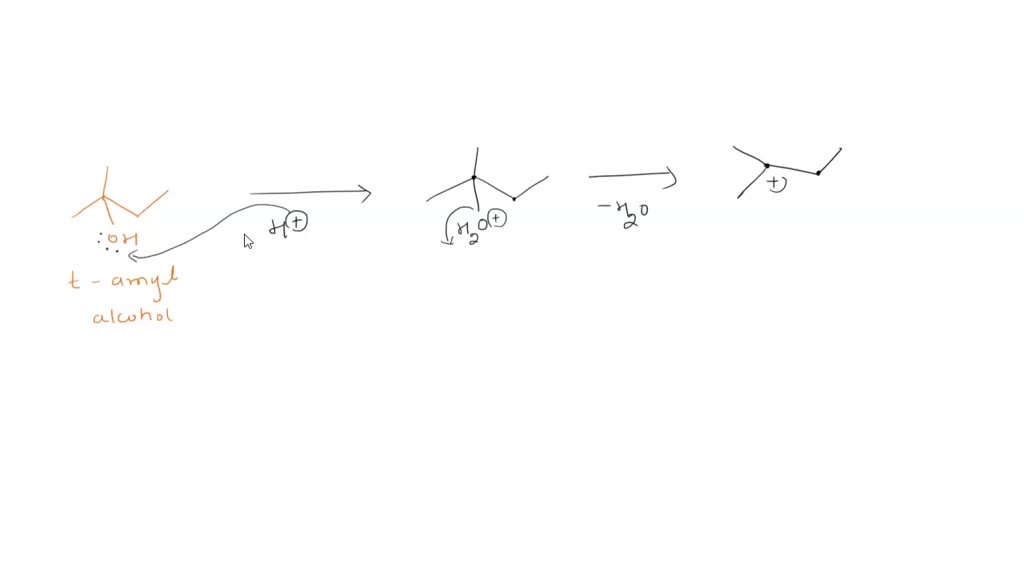
1) Show the mechanism for the conversion of 2-methyl-2-butanol into 2 -chloro-2-methyl butane. Remember to show the movement of electron pairs. 2) What type of reaction could occur if the alcohol and H
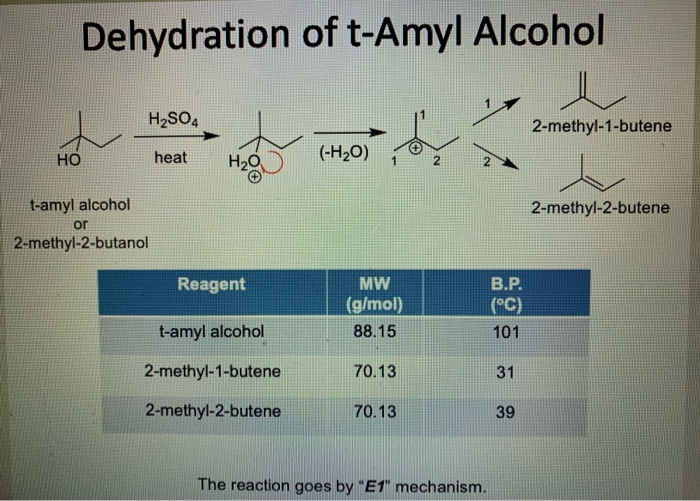
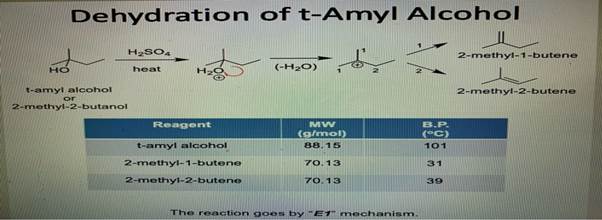
SOLVED: Draw mechanisms for formation of possible alkenes obtainable from t-amyl alcohol. Explain why 2-methyl-2-butene is the alkene that is obtained.
PDF) Etherification rates of 2-methyl-2-butene and 2-methyl-1-butene with ethanol for enviromentally clean gasoline production

OneClass: Ifthe reaction of 2 methyl 2-butanol + HCl to give 2-chloro-2-methylbutane is conducted in ...
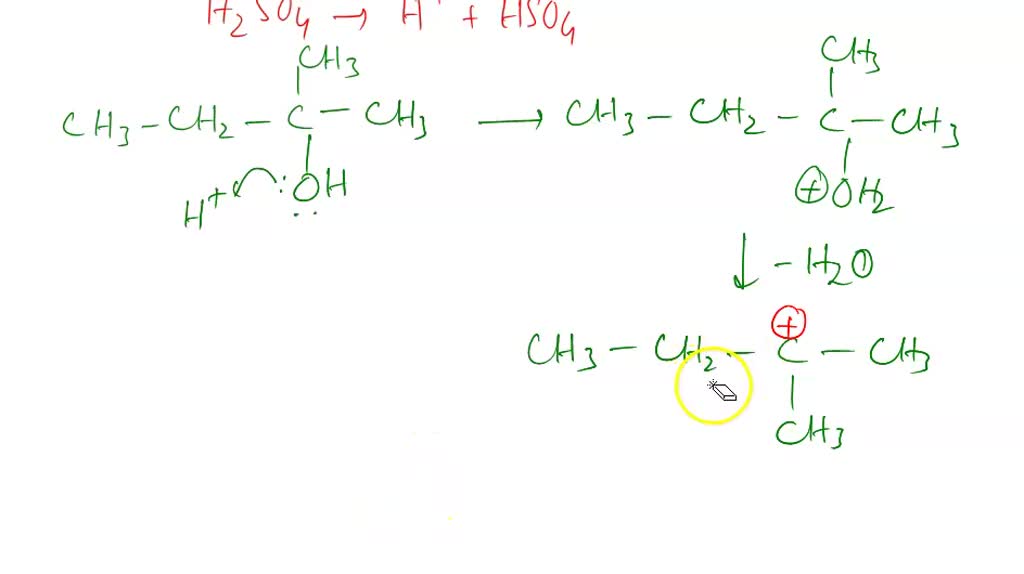
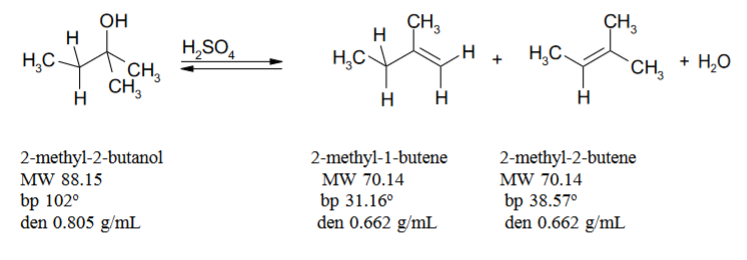
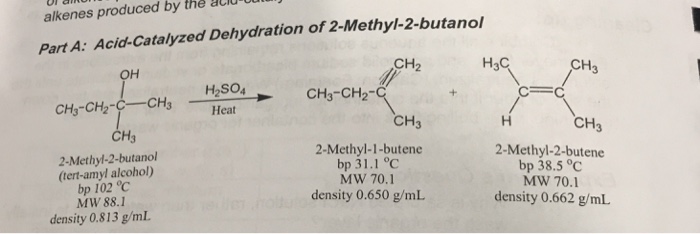
SOLVED: Dehydration of 2-methyl-2-butanol forms a major (2-methyl-2-butene) and a minor (2-methyl-1-butene) organic product. What are the mechanisms for the formation of each?
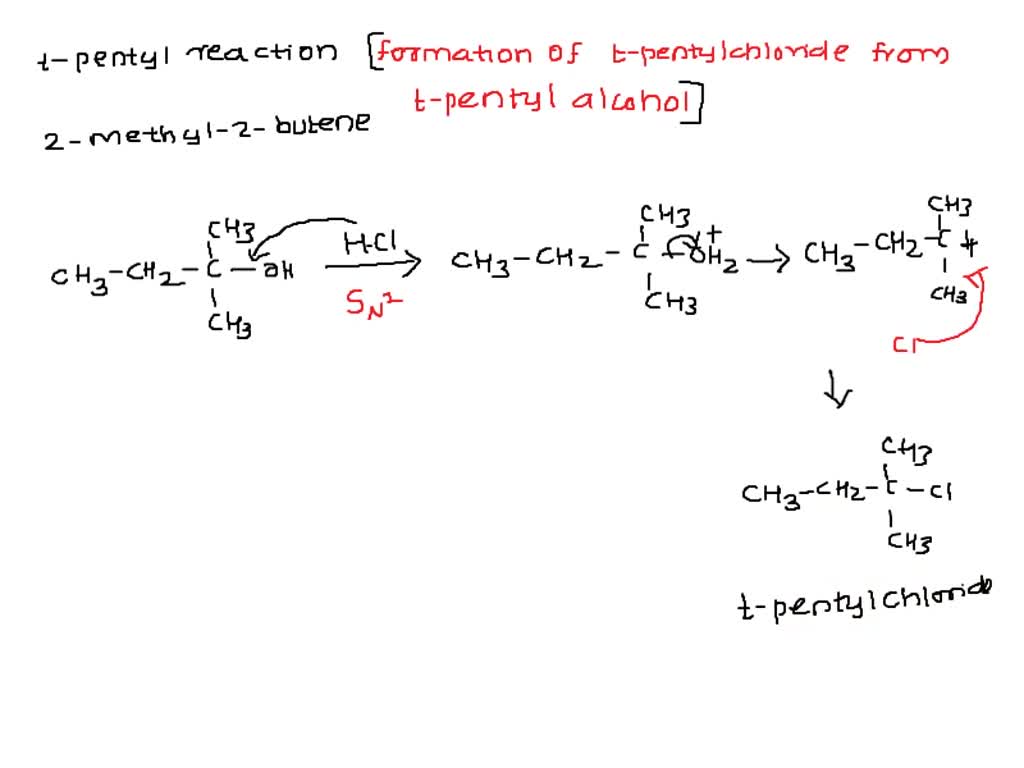
SOLVED: 3 . Some 2-methyl 2-butene may be produced during the t-pentyl reaction Explain this mechanistically.

What is the major product obtained from hydroboration-oxidation of 2-methyl- 2-butene? | Homework.Study.com